Plastics solutions for medical technology
Ready for new possibilities
Ensinger one-stop shop for medical technology
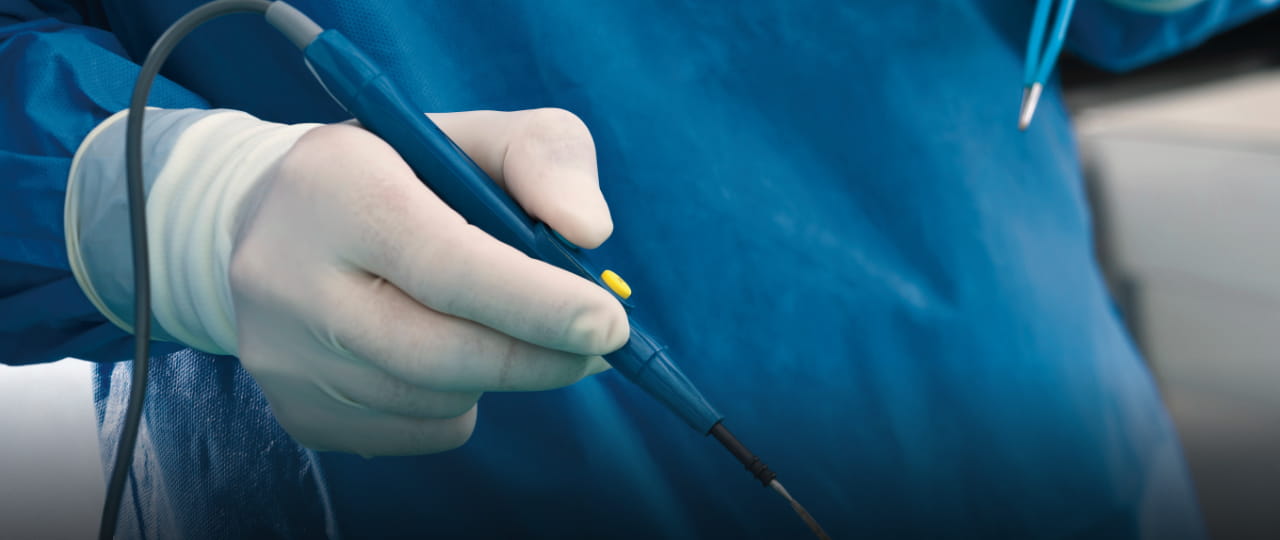
Achieving the best possible quality of life and mobility into old age, the desire to live as pain-free a life as possible from childhood - the major trends in the medical industry are leading to an increase in surgical interventions and new treatment options. This is increasing the price and cost pressure on both healthcare systems and medical device manufacturers.
At the same time, efforts are being made to improve patient safety, for example by requiring unannounced audits, intensifying clinical evaluation and introducing additional testing procedures for certain higher class medical devices.
This includes increased consideration of physiological safety, even for short-term patient contact.
In order to meet these requirements in the future, the development of innovative products is of great importance for medical device manufacturers. Product safety, patient benefit and cost control must be at the forefront.
More than Just one solution
As a full-service provider for solutions in medical technology, Ensinger supports manufacturers of medical products with alternative but proven materials and process technologies. We cover the entire value chain in-house, offering our customers the highest level of quality, safety and flexibility. Together with our customers, we develop and manufacture products that are precisely tailored to their needs. The safety of the patient comes first, both for the selection of physiologically safe materials as well as for the evaluation respectively testing of the biocompatible properties. Ensinger medical grade plastics are marked with the trade name abbreviation MT respectively MED for Compounds.
Our comprehensive portfolio of Ensinger Medical Grade (MT, MED) plastics offers designers a wide range of options for developing innovative medical products with high levels of safety and functionality. Our wide range of manufacturing processes, such as extrusion, machining, injection molding, profile extrusion and compounding, also offer our customers maximum flexibility. From material to finished part, we offer the highest quality along the entire value chain and provide the entire portfolio of solutions to the finished part in-house.
Within the Ensinger medical grade (MT, MED) plastics, high compliance standards apply with regard to documentation, traceability and notification of changes. This also enables consistent knowledge and data transfer towards authorities for the approval of products worldwide.